Carboxylic acids (-COOH) are functional groups composed of one carbonyl and one hydroxyl group.
A carboxyl group (COOH) is a functional group consisting of a carbonyl group (C=O) with a hydroxyl group (O-H) attached to the same carbon atom. Carboxyl groups have the formula -C(=O)OH, usually written as -COOH or CO2H. Carboxylic acids are a class of molecules which are characterized by the presence of one carboxyl group. As proton donors, carboxylic acids are characterized as Brønsted-Lowry acids. Acids with two or more carboxylic groups are called dicarboxylic, tricarboxylic, etc. Salts and esters of carboxylic acids are called carboxylates. Carboxylate ions are resonance-stabilized. This increased stability leads to increased acidity compared to that of alcohols. Generally, in IUPAC nomenclature, carboxylic acids have an “-oic acid” suffix, although “-ic acid” is the suffix most commonly used.

Nomenclature
When naming compounds with a carboxylic acid group present, it is appropriate to replace the ending (the -e, for example) with -oic acid. The acidity of the carboxylic acid comes from the ability to donate the proton on the -OH substituent.
Physical Properties
Due to the strong hydrogen-bonding, two important factors with carboxylic acids are that they have a high boiling point but are also soluble in water and other organic solvents such as toluene and ethanol. Carboxylic acids are also highly reactive to two factors: the two electron-rich oxygens in the carbonyl and alcohol portion of the functional group, and the electron-poor carbon between the carbonyl alcohol group, which is a desirable attack location for nucleophiles.
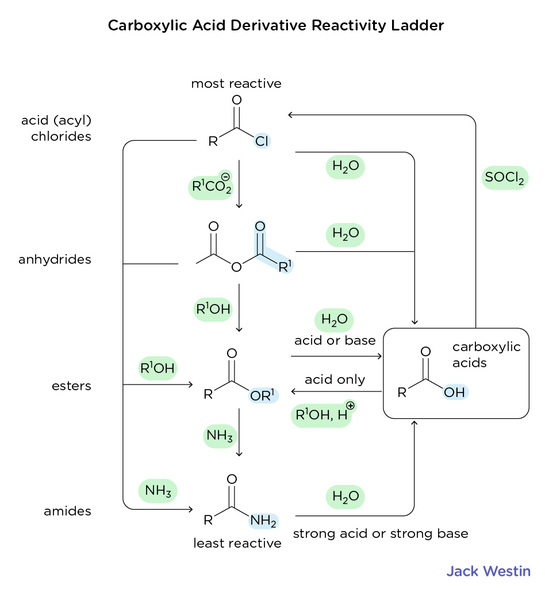
Practice Questions
Khan Academy
MCAT Official Prep (AAMC)
Official Guide C/P Section Passage 3 Question 10
Chemistry Question Pack Question 15
Key Points
• Carboxylic acids are used as precursors to form other compounds such as esters, aldehydes, and ketones.
• Carboxylic acids can exhibit hydrogen bonding with themselves, especially in non-polar solvents; this leads to increased stabilization of the compounds and elevates their boiling points.
• Since they contain both hydroxyl and carbonyl functional groups, carboxylic acids participate in hydrogen bonding as both hydrogen acceptors and hydrogen donors.
• Compounds with a carboxylic acid group present adopt the suffix -oic acid in their nomenclature.
• Carboxylic acids have a high boiling point and have an easily reactive electron-poor carbon.
Key Terms
Brønsted-Lowry acids: A chemical species that donates one or more hydrogen ions in a reaction.
Carbonyl: Group containing double-bonded carbon and oxygen, written as C=O.
Alcohol: Group containing a hydroxyl, written as -OH.
Ester: A compound most often formed by the condensation of an alcohol and an acid, with elimination of water. It contains the functional group C=O joined via carbon to another oxygen atom.
Nitrile: Any of a class of organic compounds containing a cyano functional group (-C≡N).
Olefin: Any of a class of unsaturated, open-chain hydrocarbons such as ethylene; an alkene with only one carbon-carbon double bond.









